OBJECTIVE for PQ of Bio Dot Dispenser
To determine that the PQ of Bio Dot Dispenser perform as intended by repeatedly running the system on its intended schedules and recording all relevant information and data. Results must demonstrate that perform consistently meets per-determined specifications under normal condition, and where appropriate the worst case situations.
TABLE OF CONTENT
| PARTICULARS | PAGE NO. |
| TITLE PAGE | |
| TABLE OF CONTENT | |
| OBJECTIVE | |
| PROJECT BACKGROUND & SYSTEM DESCRIPTION | |
| PRE-APPROVAL OF DOCUMENTS | |
| QUALIFICATION PROCEDURE | |
| REFERENCES | |
| RESULT | |
| DEVIATION | |
| SUMMARY | |
| CONCLUSION | |
| RECOMMENDATIONS | |
| ATTACHMENTS | |
| POST-APPROVAL OF DOCUMENTS | |
| LIST OF ABBREVIATIONS | |
SCOPE
The scope of this qualification document is to perform the Performance qualification to verify the installed & operational components as described in IQ & OQ.
This document is applicable for Bio-Dot Dispenser located at Medical Devices Area.
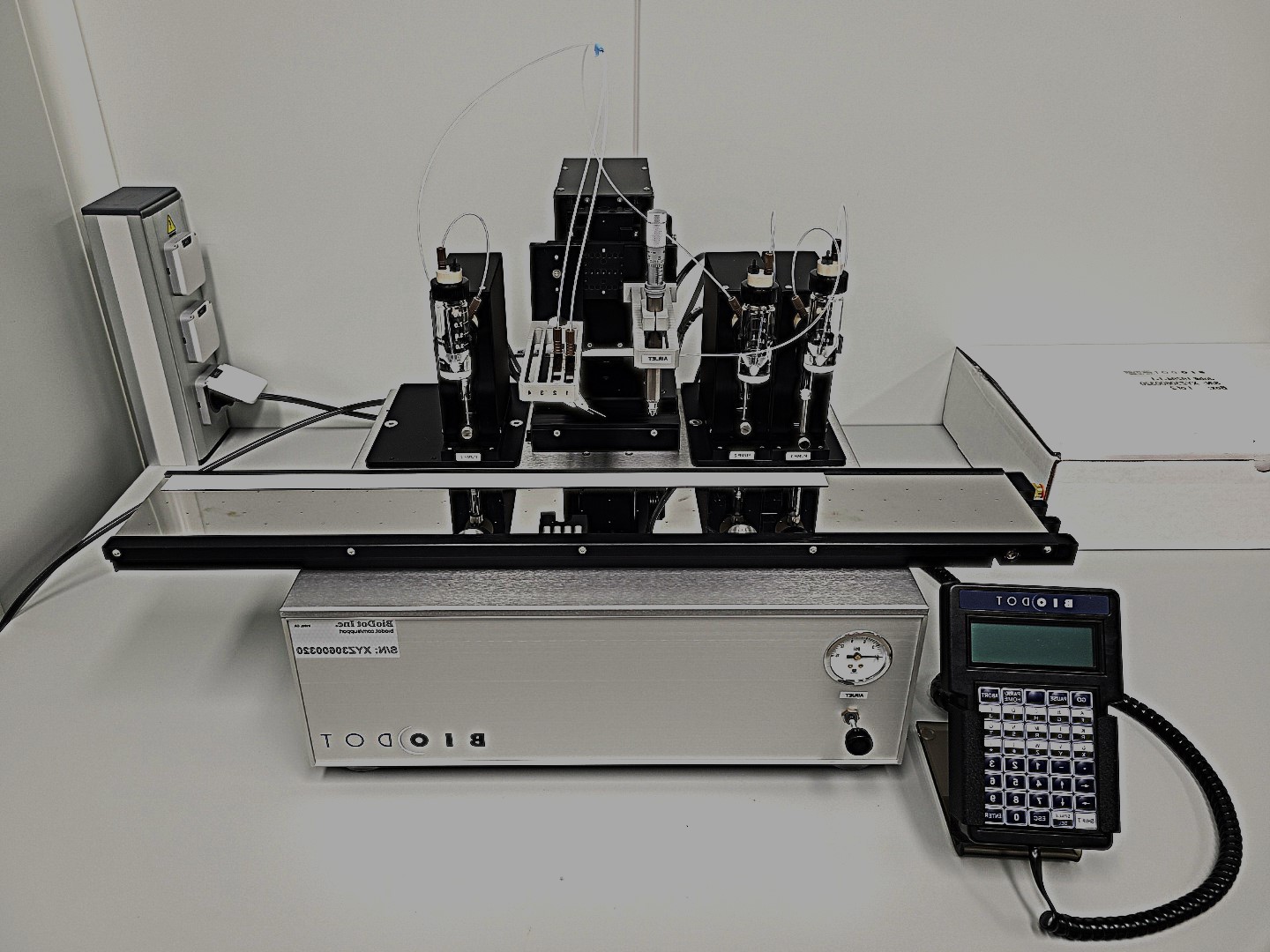
PROJECT BACKGROUND & SYSTEM DESCRIPTION
Bio Dot Dispenser is that work with the syringe pump to make up the complete dispenser kit with a high resolution syringe pump to dispense precise amount of reagent. Syringe size available 250 micro litre.
PRE APPROVAL OF DOCUMENTS
Prepared by
| Designation | Name | Date | Signature |
| Executive/Officer Production |
Reviewed By
| Designation | Name | Date | Signature |
| Head Engineering | |||
| Head Production |
Approved by
| Designation | Name | Date | Signature |
| Head QA |
SCOPE OF SUPPLY:
BRIEF PROCESS DESCRIPTION:
Bio Dot dispenser is designed to dispense reagents onto membrane for the development and manufacture of rapid diagnostic tests. Each system can be configured to dispense either via contact (front line), non- contact dispensing or combination. It consists of the
- Syringe pump
- Dispenser
Syringe Pump:- The syringe pump displaces the fluid towards the dispensers by utilizing a positive displacement pump, a programmable lead screw and a syringe.
Dispenser:- This series of dispenser consists of two syringe pump (250 micro litre) which sprays with airstream technology with one or two syringe pump to dispense precise amounts of reagent.
REQUIRED UTILITES:
| Utility/ Parameter | Features |
| Electrical Utility | Voltage: 220 VAC ± 5% |
| Frequency: 50 Hz ± 2% | |
| Air Suply:- 40-60 PSI | |
| Phase: 1 Phase, 1 Neutral,1Earthing |
PQ of Bio Dot Dispenser PRE REQUISITES:
- The location and placement of machine shall be verified.
- The Design Qualification, Installation Qualification & Operation Qualification has been checked for meeting the requirements.
- All the equipment of the system has been identified and verified.
- The availability of engineering drawings/schematics/Operational manual has been checked.
PQ of Bio Dot Dispenser Procedure
Physical verification:
| Parameters | Acceptance Criteria | Observation | Remarks | Done by |
| Horizontal leveling of the equipment | Should be perfectly horizontal | |||
| Positioning of the equipment | It should be aligned vertically straight. There should be enough space to open guards for maintenance purposes. | |||
| Floor balancing | It should not give any vibration at any corner of the equipment and should be well placed on the floor | |||
| Identification plate | Name of the equipment and/or suppliers name to be available on the equipment | |||
| Surface finish of equipment | Should be smooth and glossy. | |||
| Any physical damage to the Equipment. | No physical scratches or damage should be observed | |||
| Identification of label. | Shall be properly Labeled. | |||
| Cleaning of the equipment. | Cleaning of the equipment shall be verified. | |||
| Verification of OQ. | OQ of the equipment shall be verified. | |||
| Availability of equipment SOP | Effectiveness of SOP shall be checked. |
Cleaning check:
| Check points | Observation | Checked By (Sign & Date) |
| Check the cleanliness of the external body of Bio Dot Dispenser. | ||
| Check the cleanliness of the Platform. | ||
| Check cleanliness of the Reagent Bottles Cleaning. | ||
| Check the cleanliness of Reagent Pump Cleaning. |
| Product Name | Batch Number | Checked By | Verified By |
Checking Points before Startup
| Check Points | Acceptance Criteria | Observations | Done By (Production) Sign/Date | Checked By (QA) Sign/Date | ||
| Check the availability of Backing Laminated card affixed Nitrocellulose membrane. | Nitrocellulose membrane should be affixed on Backing Laminate | |||||
| Check the machine for abnormal vibration. | Machine vibration should not be abnormal. | |||||
| Check the proper locking of Reagent bottles. | Reagent bottles should be properly locked & there should be no leakages or spillage. | |||||
| Check the smooth movement of the Platform. | Platform should be move smoothly. | |||||
| Status Checkup for activeness | It should be Active | |||||
| Status checkup for Shape | LINE status should be there. | |||||
| Standard length in X direction is 370mm and in Y direction is 70mm | Standard lenth should be within 70-370mm | |||||
| Ensure the dispense head is at an appropriate distance from the surface of the substrate | Dispense head to Substrate surface should be 1-3 cm | |||||
| Platform Speed should be 01-300 (mm/Sec) | 01-300 mm/sec. | |||||
| Polarization YES/NO | When line to be drawn only in one direction set to “YES” & for multiple lines set to “NO” | |||||
| Status Checkup for activeness. | It should be ACTIVE | |||||
| Check for Dispense Rate. | Dispense rate should be 0.07 µL/mm | |||||
| Check for Syringe Size as per process. | Syringe size should be 50 µL, 100 µL, 250 µL, 500µL | |||||
Product Verification
| Product Name | Batch Number | Observation | Checked By | Reviewed By |
Acceptance Criteria:
- Equipment/ Machine should be operating as defined in the standard operating procedure, if differs, then record deviation & check the performance of machine.
- Operation should be smooth and there should not be any abnormal observation during the operation.
PERFORMANCE QUALIFICATION REPORT:
_______________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
REFERENCES:
Performance Qualification SOP:-
RESULT:
_____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Checked By (Sign & date):________________
DEVIATION/CHANGE CONTROL
| S.NO | Deviation |
Checked By (Sign & date):________________
CONCLUSION:
_______________________________________________________________________________________________________________________________________________________________________________________________________________
Done By (Sign & date):________________
RECOMMENDATIONS
_______________________________________________________________________________________________________________________________________________________________________________________________________________
Done By (Sign & date):________________
ATTACHMENTS:
| Document name | Reference No. | Checked by Sign & Date |
POST APPROVAL OF DOCUMENTS
Prepared by
| Designation | Name | Date | Signature |
| Officer/Executive Production |
Reviewed By
| Designation | Name | Date | Signature |
| Head Engineering | |||
| Head Production |
Approved by
| Designation | Name | Date | Signature |
| Head QA |
LIST OF ABBREVIATIONS
| PQ | Performamnce Qualification |
| V | Volt |
| Hz | Hertz |
| µL | Micro Litre |
| mL | Mili liter |
| Cm | Centi Meter |
| Mm/sec | Mili mitre per second |